Background:First-line (1L)standard of care (SOC) options for patients with FL include an anti-CD20 monoclonal antibody with or without chemotherapy, and second-line (2L) therapeutic options are similar to 1L. There is no defined SOC for patients who require treatment in third, fourth, and later lines of therapy (LOT). Additionally, patients with high-risk FL who relapse ≤ 24 months after frontline treatment (POD24) have limited treatment options that can provide a durable response. Recently, novel treatments such as CAR T cell therapies and T cell engager regimens have been shown to improve efficacy outcomes in third line or later (3L+) and POD24 populations. We conducted an SLR of all relevant clinical evidence to evaluate the efficacy and safety of available therapies used in the treatment of adult patients with R/R FL in 3L+, fourth line or later (4L+), and 2L+ POD24 settings. We also aimed to conduct meta-analyses to derive pooled effect estimates for response and survival outcomes in these settings.
Methods: Publications from 01/01/1998 to 09/17/2022 were identified using Ovid MEDLINE ®, Embase, and Cochrane Central Register of Controlled Trials, with supplemental hand searches of key conferences and bibliographies of recent relevant SLRs. Study eligibility was assessed according to prespecified population, intervention, comparator, outcome, and study design criteria. Studies were eligible if they were in English and enrolled patients with R/R FL (grade 1‒3A) in 3L+, 4L+, and POD24 settings. Eligible treatments included CAR T cell therapies (axicabtagene ciloleucel, lisocabtagene maraleucel, and tisagenlecleucel), T cell engagers (mosunetuzumab, glofitamab, epcoritamab, odronextamab), phosphatidylinositol 3-kinase (PI3K) inhibitors (copanlisib, duvelisib, idelalisib), HSCT, yttrium-90 (90Y) ibritumomab tiuxetan, tazemetostat, and conventional therapies (immunochemotherapies, single- or multiagent chemo- or immunotherapies, and alkylating agents). Outcomes of interest were OS, PFS, ORR, CR rate, PR, duration of response, and time to next treatment. Independent review committee (IRC) data were extracted when both IRC- and investigator-assessed data were reported. Pooled estimates for ORR, CR rate, OS, and PFS across all treatments were calculated among 3L+, 4L+, and POD24 patient populations. For POD24 studies, we combined POD24 definitions (ie, definitions with respect to POD24 from frontline therapy or baseline) and POD24 patients across LOTs (ie, presenting in 2L+). For each outcome in 3L+, 4L+, and POD24 settings, ≥ 4 sufficiently similar studies were required for a meta-analysis to be deemed feasible.
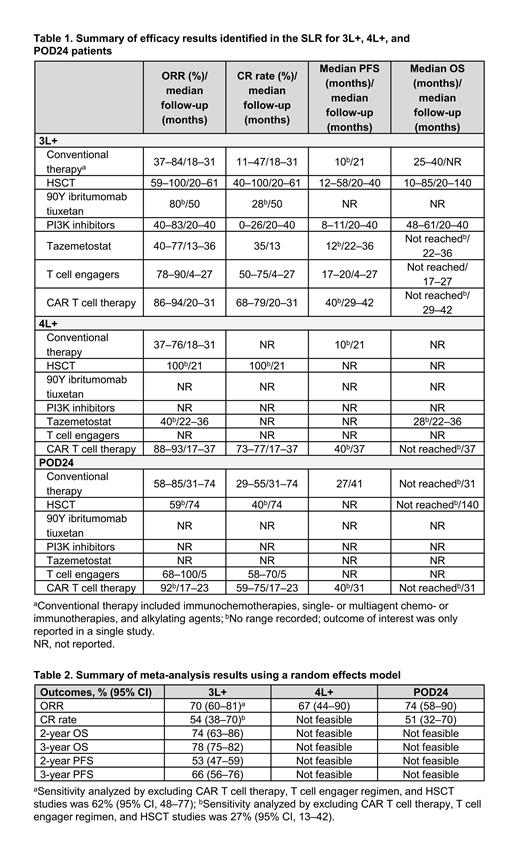
Results: Of 5250 records screened, 112 were included, representing 60 unique studies. Of 60 studies, 33 (55%) were retrospective or prospective observational, while 27 (45%) were clinical trials (randomized, nonrandomized, and single arm). There was significant heterogeneity in patient populations and baseline characteristics across studies. Most studies (n = 48; 80%) reported findings for 3L+ FL, with limited evidence for 4L+ and POD24 patients. Results for ORR, CR rate, PFS, and OS by treatment type across 3L+, 4L+, and POD24 patients are presented in Table 1. ORR and CR rate data were highly variable depending on treatment used and length of follow-up period in the study. Across 3L+, 4L+, and POD24 patients, the highest ORR and CR rate ranges were observed for CAR T cell therapies, T cell engagers, and HSCT. Median PFS was highest for HSCT, T cell engagers, and CAR T cell therapy in the 3L+ setting and was not reported or not reached for HSCT and T cell engagers in 4L+ and POD24 settings. Median OS was not reached for patients receiving T cell engagers in the 3L+ setting or those receiving CAR T cell therapy in the 3L+, 4L+, and POD24 settings. Various meta-analyses including all treatments were considered feasible for 3L+, 4L+, and POD24 populations (Table 2). For ORR and CR rate in the 3L+ population, sensitivity analyses excluding CAR T cell therapies, T cell engager regimens, and HSCT were feasible and showed lower ORR and CR rate when these therapies were removed.
Conclusions: This SLR demonstrated an evolving FL treatment landscape, with new agents such as CAR T cell therapies and T cell engagers exhibiting potential for improving effectiveness of treatment for patients in 3L+, 4L+, and 2L+ POD24 populations.
Disclosures
Craigie:Bristol Myers Squibb: Consultancy. Ramirez:EVERSANA: Current Employment. Rosta:EVERSANA: Current Employment. Desai:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Fasan:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company; Sanofi Genzyme: Speakers Bureau; Oncopeptides: Other: Advisory Board, Speakers Bureau. Farazi:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Kumar:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal